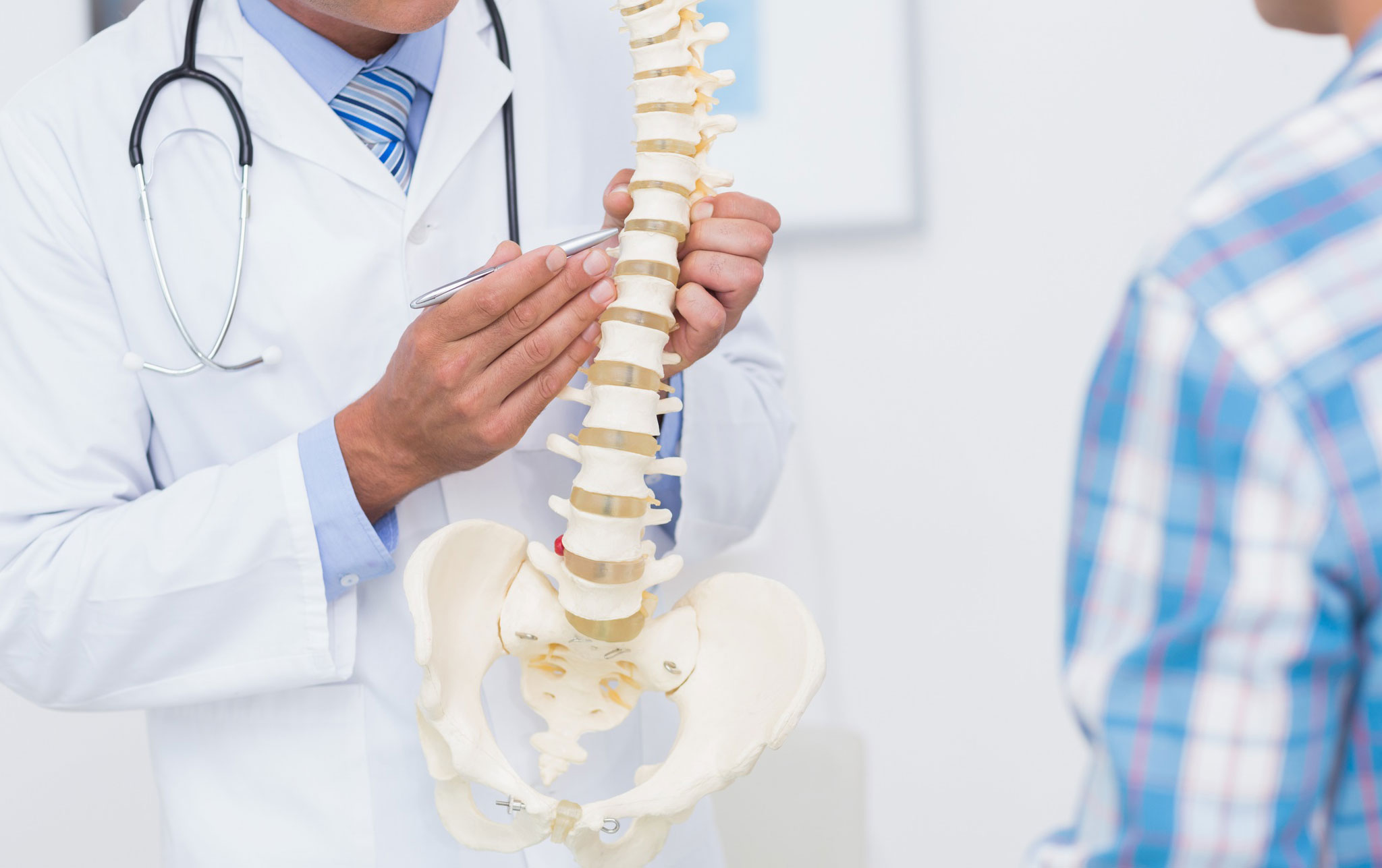
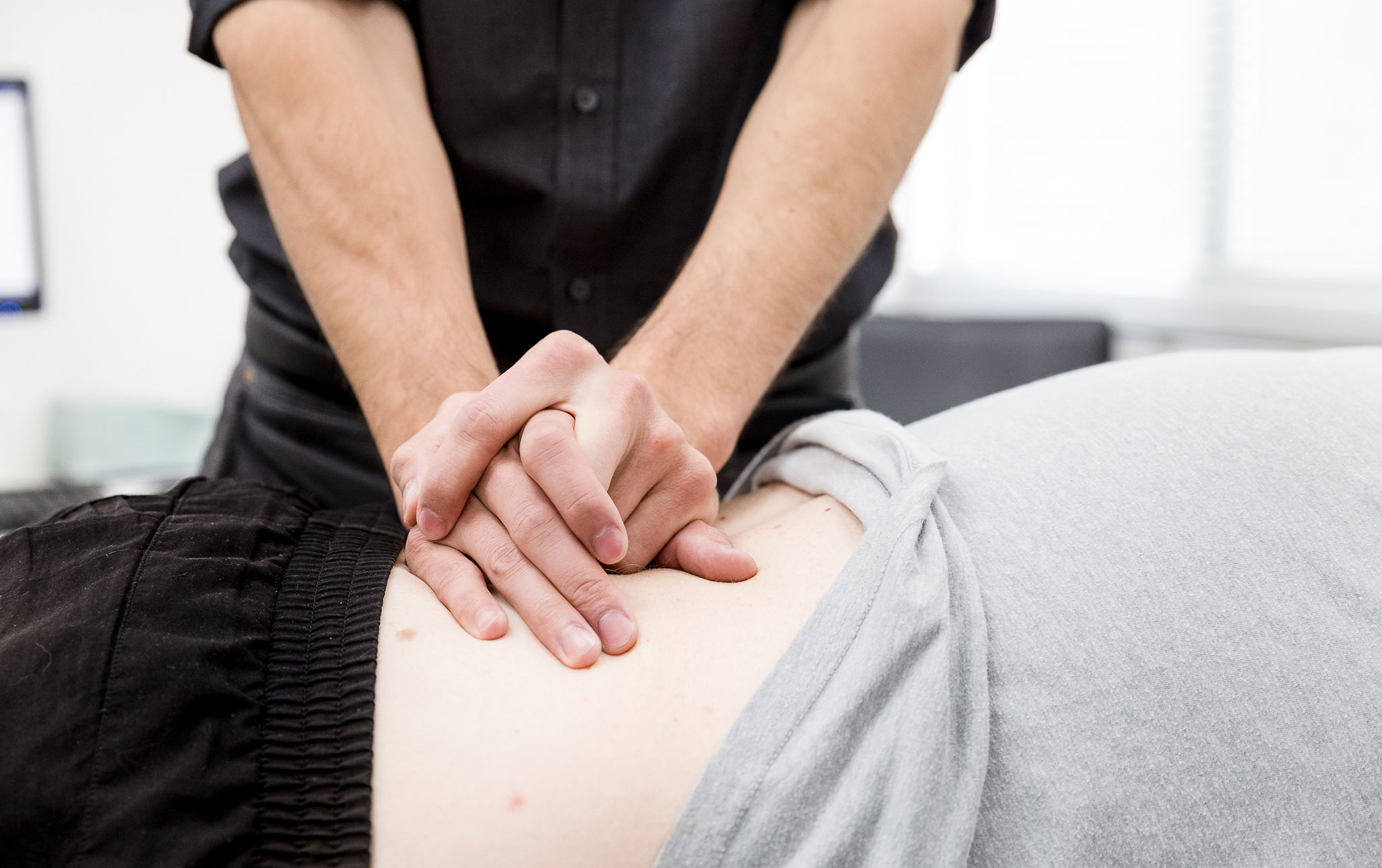
Qualified Staff
Highly qualified medical and support staff who provide safe and effective care. Our patient’s experience at CBC is of upmost importance.
Shared Decision Making
We offer both conservative and surgical treatment options, and recognize each patient is an individual regardless of diagnosis. Our providers employ a shared decision making process with each patient to determine which treatment option is best for them.
Quality Services
We provide a variety of high quality treatment options, many of which are offered on site. In-office therapeutic spinal injections take place in our procedure suite, and advanced non-ionizing imaging is obtained in our gait lab.
ASK US ABOUT GETTING A CT MEDICAL MARIJUANA CARD!
Our physicians can initiate your application by certifying that you have a medical condition that qualifies you for a medical marijuana registration certificate.
For adults, debilitating conditions include:
Cancer, Glaucoma, Positive status for HIV or AIDS, Parkinson’s disease, Multiple Sclerosis, Damage to the nervous tissue of the spinal cord or irreversible spinal cord injury with objective neurological indication of intractable spasticity, Epilepsy, Cachexia, Wasting syndrome, Crohn’s disease, Post-traumatic stress disorder, Sickle cell disease, Post-laminectomy syndrome with chronic radiculopathy, Severe psoriasis and psoriatic arthritis, Amyotrophic lateral sclerosis, Ulcerative colitis, Complex regional pain syndrome, types 1 and 2, Cerebral palsy, Cystic fibrosis, Terminal illness requiring end-of-life care, Uncontrolled intractable seizure disorder, Spasticity or neuropathic pain associated with Fibromyalgia, Severe rheumatoid arthritis, Postherpetic neuralgia, Hydrocephalus with intractable headache, Intractable headache syndromes, Neuropathic facial pain, Muscular dystrophy, Osteogenesis imperfecta, Chronic neuropathic pain associated with degenerative spinal disorders.
Certification is good for a year and should be renewed in the 11th month to avoid a lapse in coverage.
Helping You Live Your Life More Comfortably
If you are one of the many people suffering from neck pain, chronic lower back pain, scoliosis, sciatica or another spine-related condition, you will be in great hands at Connecticut Back Center (CBC). CBC was founded by Jesse Eisler, MD, PhD, in 2005 and serves the Greater Hartford and Central Connecticut area. Our mission is to provide accurate, responsive and effective spinal care with comprehensive non-surgical and surgical treatments for the full spectrum of spinal disorders. Our goal is always to reduce your pain, increase your flexibility and help you resume an active life after treatment.
When spine surgery is your best or only treatment option, we will employ the latest state- of-the-art techniques, including degenerative conditions of the spine, such as cervical spinal stenosis, lumbar spinal stenosis, cervical or lumbar disc herniation, and scoliosis or adult deformity of the spine. We have specific expertise in the management of osteoporotic spines and we treat spinal trauma and injuries to the spine. We also provide sciatica treatment for sciatic nerve pain.
Your treatment for degenerative conditions of the spine may include minimally invasive surgery, traditional open and spinal fusion surgery procedures, motion preserving disc arthroplasty/replacement or dynamic stabilization of the spine. We may also recommend chiropractic treatments and physical therapy, which have been proven effective for many neck and back conditions. We believe that increasing a patient’s core strength is always beneficial.
We welcome patients from all areas of Connecticut, and are often able to accommodate visit times based on patient preferences. Please contact us if you have been searching for a “back specialist near me.”
Our Staff

Jesse Eisler
MD, PhD
After completing a combined MD and PhD program as part of the Medical Scientist Training Program sponsored by the National Institute of Health..

Andrew Gregory
NP-C
Dr. Andrew Gregory is a graduate of Samford University and is certified as a Family Nurse Practitioner by the American Academy of Nurse..
What Our Patients Say About Us
“Thank you very much for giving me your time and expertise. I feel blessed to have found you and your staff”
“Thank you for your guidance, expertise and oversight in helping me recover from a very painful, stressful and challenging medical event”
“I want to express my thanks for all of your care, concern and most important, for taking away all of my pain”
Our Blogs
Congratulations to Dr. Jesse Eisler on completing the FIRST dualPortal® case in Connecticut, performed in Hartford CT!
I’ve been observing the spine endoscopy field for over 10 years and I’m so impressed with the progress being made by surgeons and device companies and appreciate the work Amplify Surgical, Inc. and Andy Choi are doing to make this effective and cutting-edge technique...
In October, CT Back Center wears PINK! in support of Breast Cancer Awareness Month!
October 16th is World Spine Day!
October 16th is World Spine Day! "Taking place on October 16 each year, World Spine Day highlights the burden of spinal pain and disability around the world. World Spine Day highlights the importance of spinal health and well-being. Promotion of physical activity,...
Connecticut Back Center offers a wide range of treatment options, find out which one is best for you!